Past Projects
PHYTOPLANKTON COMMUNITY STRUCTURE AND CARBON EXPORT IN THE NORTH PACIFIC
As a graduate student in the Quay Lab at the University of Washington, I participated in two trans-Pacific research cruises (summer and fall 2011, see image) on the OOCL container ship Tokyo to gather baseline data on the temporal and spatial variability in rates of primary production, phytoplankton community structure (in collaboration with the Armbrust Lab), and carbon export in the subtropical and subarctic North Pacific.
Results from this work can be found here:
Palevsky HI, Quay PD, Lockwood DE, & Nicholson DP. (2016) The annual cycle of gross primary production, net community production and export efficiency across the North Pacific Ocean. Global Biogeochemical Cycles29
Online
TARGETED METABOLOMICS IN GIANT CLAMS AND REEF-BUILDING CORALS
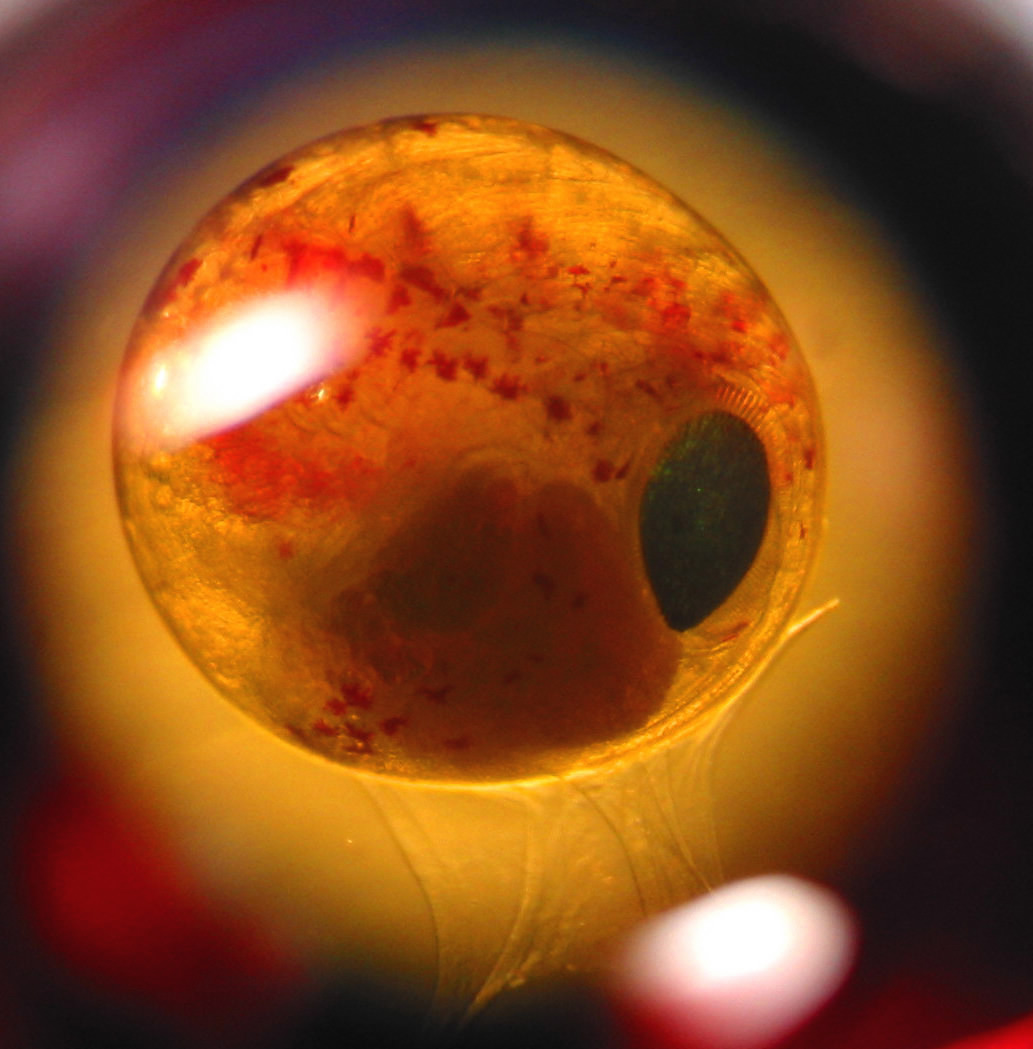
Glycine betaine. One of the most abundant betaines in marine organisms.…
Glycine betaine. One of the most abundant betaines in marine organisms.
When organisms are faced with osmotic stress they often produce compounds known as osmolytes to help reduce osmotic pressures and thereby protect cells from damage from dessication or over-hydration. One class of purported osmolytes are betaines and in particular, the compound glycine betaine (see image).
Interestingly, glycine betaine has also been implicated in plants to play a role in stabilizing chloroplast photosytem II complexes leading to reduced oxidative damage during periods of intense photosynthesis. Glycine betaine (and other related compounds) have been recently reported in corals and are associated with both the zooxanthellae and cnidarian hosts. Although more reserach needs to be done to ascertain the exact functional role of betaines in corals, their presence suggests that corals may be using betaines in a dual capacity - both as osmolytes and as photoprotectants
As an undergraduate, I worked in the Hill Lab using a targeted metabolomics approach to identify and quantify betaine compounds within samples of giant clam mantle (siphonal and byssal), adductor mussel, and gill. Several betaines were found to be present in extremely high abundance in giant clam tissues. As in corals, this suggests that betaines may be playing a functional role in tridacnid clams - either as osmolytes, or perhaps as stabilizing agents for the photosystem II complex of symbiotic zooxanthellae.
Results from this work can be found here:
Hill RW, Armstrong EJ, Florn AM, Li Chao, Walquist RW, Edward A. (2017) Abundant betaines in giant clams (Tridacnidae) and western Pacific reef corals, including study of coral betaine acclimatization.
Marine Ecology Progress Series. 56
Online
GULF OF MAINE CYANOHAB MONITORING PROJECT
As an undergraduate student, I interned in the Anderson Lab at Woods Hole Oceanographic Institute(WHOI) where we used a Fluorescent-In-Situ-Hybridization (FISH) technique to identify cyanobacterial species which secrete potent toxins that are harmful to humans and other organisms.
Conceptual cartoon of fluorescent in-situ hybridization technique on the left. On the right, examples of labelled and unlabelled cyanobacterial cells. The goal is to develop fluorescent probes with are specific to some species (e.g. toxic Microcystis spp.) while non-reactive to others (e.g. non-toxic Anabaena spp.) as shown here.
FISH is a staining technique that allows for labelling of cells containing a specific sequence of either DNA or RNA. A fluroescent molecule is attached to a small nucleotide primer which is complementary to the sequence of DNA or RNA of interest in the cell (see image below). When the primer encounters this target sequence it binds, bringing the fluroescent molecule along with it and thereby "labelling" the cell. If enough primer binds inside a cell, that cell can be identified under a fluorescent imaging microscope (see below).
The goal of this project was to design RNA-binding probes from available 16s-rRNA data for three most important toxic cyanobacteria species in the Gulf of Maine (Microcystis aeruginosa, Anabaena flos-aquae, and Cylindrospermopsis raciborskii). Using FISH, we were able to clearly label potentially toxic cells, thereby allowing for quick and sure identification of dangerous cyanobacteria. As part of this research, I successfully developed two species-specific rRNA probes. Work on this project continues in the Anderson lab with the ultimate goal being the development of a cyanobacteria-sensing instrument that can be permanently moored in the Gulf of Maine, alerting coastal managers to the presence and abundance of toxic cyanobacterial blooms in real-time.
Conceptual cartoon of fluorescent in-situ hybridization technique on the left. On the right, examples of labelled and unlabelled cyanobacterial cells. The goal is to develop fluorescent probes with are specific to some species (e.g. toxic Microcystis spp.) while non-reactive to others (e.g. non-toxic Anabaena spp.) as shown here.